HeartSave series
What does the abbreviation 'AED' stand for?
How many electrodes are included in one package?
What is the prescribed interval for the TSC (technical safety check) for the AED?
How long is the warranty period for the AED?
I am interested in purchasing an AED – who can advise me and prepare a quote?
Can anyone buy an AED?
Are your devices really 'Made in Germany'?
What is the maximum energy your AEDs can deliver?
What is the shelf life of the AED electrodes?
Can I still use the electrodes after the expiration date?
Why do the electrodes have an expiration date at all?
Who is permitted to use an AED?
Don't I need training before I use an AED?
After buying an AED, who will explain to me how it works? Do you offer any training course for that?
Is the training voluntary or mandatory?
Do you offer online training?
What are the technical requirements for online training?
Do I have to repeat the AED training on a regular basis?
Do you also offer training material?
Do your AEDs operate according to the current guidelines?
What is sudden cardiac death?
What is defibrillation?
Can an AED be used for children?
How should an AED be stored?
What is a defibrillator?
What does a defibrillator do?
Can I harm or injure the patient with a defibrillator?
Can anyone use a defibrillator?
What is a technical saftey check / TSC?
My AED started talking after I removed the yellow transport strip, what should I do?
Consumables & Accessories
Which SavePads do I need?
I would like to order SavePads/ a battery (consumables), who do I turn to?
What information do I need to order consumables?
Where can I find the SN of my device?
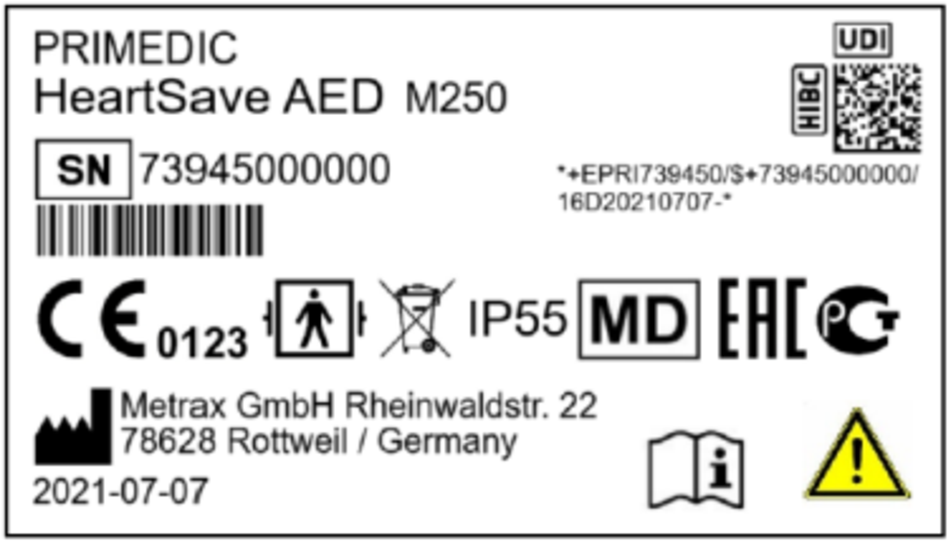
Each AED of the HeartSave series has a type plate on the back of the device, which states the device type, the serial number of the AED as well as the production date.
What is the best way to disposed of SavePads?
The LED lights on my Rotaid wall box are not working, what should I do?
The heater of my Rotaid wall box is not working, what should I do?
Service
How can a battery be disposed?
Who do I contact if my device has a defect and needs to be repaired?
I sent in my device. It was checked by a service technician and a cost estimate was drawn up. However, I don't want to have it repaired, what costs will I incur?
Do you also offer an on-site repair service?
I would like to send in my device for a STC, who do I turn to?
I would like to carry out an update to the latest ERC guidelines for my AED. Who do I need to contact?
What happens when the new guidelines are published?
Is there a matching guideline update for my (older version) AED then?
How is a guideline update performed?
Vigilance
How and where can I report incidents related to the AEDs?
Who do I contact if my AED did not work as it should in an emergency?
What do I do if a patient or aider is harmed during treatment?
